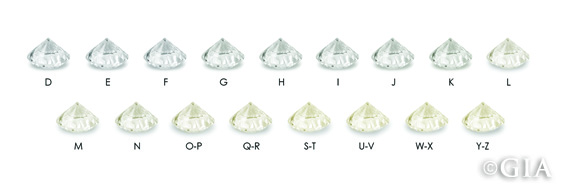
Many people know that diamonds typically come in a range of colors from D to Z on the GIA color-grading scale. However, most people don’t know how the naked eye sees color in a diamond.
When visible white light enters a diamond, the gem absorbs some of the wavelengths, while transmitting wavelengths to the viewer’s eye. This process is called selective absorption, and it determines the color of any material, including diamonds.
If little or no color is absorbed by an object, then it appears colorless or white. If the entire spectrum of colors is absorbed, the object will appear black, with an infinite number of possibilities in between.
In other words, selective absorption is the very process that determines the color of your diamond. In diamonds, the presence of nitrogen atoms (or other atoms of impurity) determines the level of absorption, and therefore the color of the diamond. A dog, because its eye processes wavelengths differently than a human, would see a completely different color in the diamond than you would.
Custom Field: Array